Please Note: These laws are theoretical. They only work on "ideal" gases. In terms of scuba diving, they are good enough for use. However, when you have much larger variables, you may encounter a noticeable difference in what should happen according to these laws, and what actually does happen.
Boyle's Law:
Boyle's law states that the more pressure is added to gas, the less volume
it will have. Specifically, it says P*V = Constant. This means that
theoretically, the pressure of one gas times the volume of the same gas is
always a constant value(NASA, <http://www.grc.nasa.gov/WWW/K-12/airplane/boyle.html>).
As such, Pi * Vi = Pf * Vf
where i is the initial value and f is the final value after pressure change.
You can derive the following equation from this: Vf/Vi
= (Number of Feet Descended) / (33ft for freshwater or 30ft for saltwater)
+ 1. The +1 accounts for the fact that you are already under 1 atmosphere
of pressure at the surface. In plain English, this formula
means that if pressure is removed (this happens when you ascend underwater),
the volume of a gas increases; if pressure is added (this happens when you
descend underwater), the volume of gas decreases. If you graph this equation,
you will notice that as you go deeper, the ratio of volumes doesn't decrease
by as much. As such, the ratio increases by more, the higher you get. To get
a good understanding of Boyle's Law, look at the animation below.
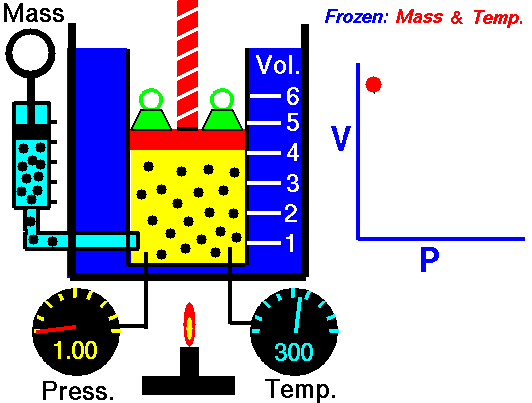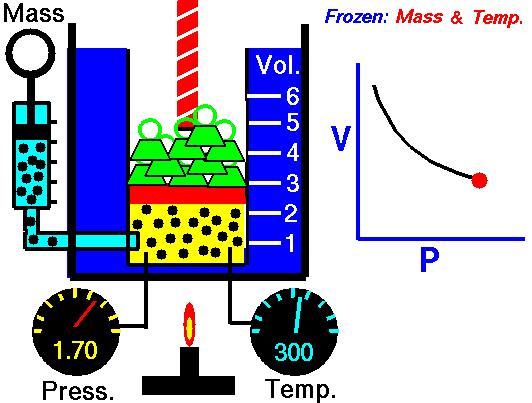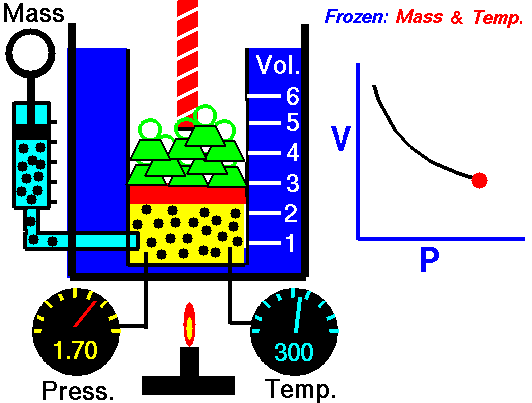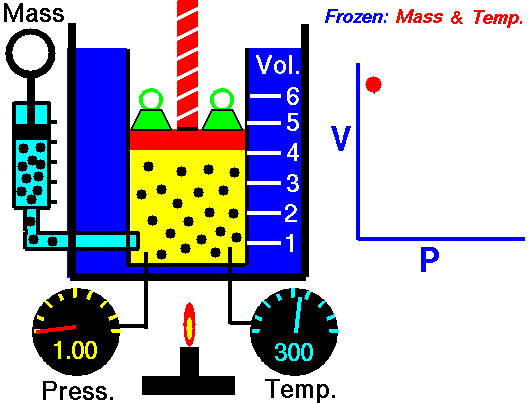
(Image Retrieved from <http://www.grc.nasa.gov/WWW/K-12/airplane/boyle.html>)
Henry's Law:
Henry's law says that the partial pressure of a gas dissolved in a solution
is directly proportional to the partial pressure of a gas above the solution(Plambeck,
<http://www.psigate.ac.uk/newsite/reference/plambeck/chem2/p01182.htm>).
For instance, about 78% of air is made up of Nitrogen. This means that at
30,000 feet below the surface of the ocean, or at any other depth, the percent
of Nitrogen dissolved in the air (assuming you have air with you) is still 78%.
